Korean Chemical Regulation
Korea REACH (K-REACH)
What is K-REACH (Korea REACH)?
The Ministry of Environment of Korea (MoE) published the Act on Registration and Evaluation, etc. of Chemical Substances on January 1, 2015. The Act is also known as K-REACH. The recent amendment was published on April 13th, 2021.
The Act on Registration and Evaluation of Chemical Substances (known as K-REACH) was published on January 1, 2015. K-REACH aims to protect public health and the environment, which is achieved through four procedures, namely notification or registration, evaluation, authorization, and restriction of chemicals.
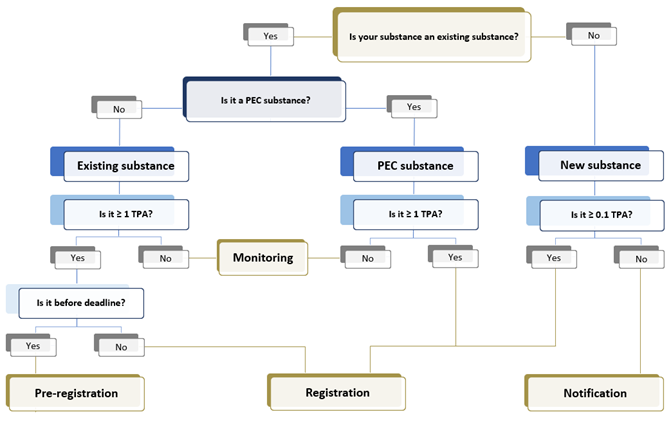
Under K-REACH, depending on if a substance is listed in the inventory, chemical substances can be divided into 3 categories, which are existing substances, new substances, and Priority Existing Chemicals (PEC) substances. Compliance procedures differ according to the substance categories and tonnage band.
There are 2 types of exemptions: confirmation required exemption and no action required exemption. The proof of exemption should be submitted for the confirmation required exemption.
Types of registration under K-REACH
New Substance
Manufacturers or importers of a new chemical substance need to register the substances prior to manufacture or import. For substance less than 100 Kg/year, it only requires notification and does not need to go through hazard evaluation.
PEC Substance
Manufacturers or importers of a PEC (Priority Existing Chemicals) substance need to register the substances prior to manufacture or import. Pre-registration of PEC substance is not possible as the transition period has already passed.
Existing Substance
For existing substances above 1 Tonne Per Annum (TPA) (excluding exempt substances), they must be registered within given grace periods. Only companies that carried out pre-notification can be entitled to the grace periods.
The deadline for existing substance registration varies based on tonnage band. After the deadline for each tonnage band, existing substances within the tonnage band cannot be pre-registered, and it should be registered prior to placing the substances in Korea.
Deadline for existing substance registration:
- 1000+ tpa: 2021.12.31
- 100-1000 tpa: 2024.12.31
- 10-100 tpa: 2027.12.31
- 1-10 tpa: 2030.12.31
K-REACH Registration Process
Other related regulations
K-BPR
The Consumer Chemical Products and Biocide Safety Management Law (known as K-BPR) concerns the placing on the market and use of hazardous consumer chemical and biocidal products, which aims to protect public health and environment from these chemicals and products. K-BPR was taken into force on January 1, 2019, and its recent amendment was promulgated on March 24, and May 26, 2020. Its enforcement will start on January 1, 2021.
Cosmetic Act
The Cosmetic Act in South Korea aims to develop the cosmetics industry and improve public health. It has been enforced since July 1, 2000, and the recent amendment came into force on April 7, 2020.
KOSHA & MSDS
MSDS is one part of Occupational Safety and Health Act (KOSHA). The purpose of this Act is to maintain and promote the safety and health of people providing labour by establishing stands on industrial safety and health.




