Turkish Chemical Regulation
Türkiye REACH (KKDIK)
What is KKDIK (Türkiye REACH) ?
KKDIK came into force as a result of the EU Adaptation policy. The first phase of pre-registration ended on December 31st, 2020. The registration phase then officially started the next day (January 1st, 2021). By complying with KKDIK, exporters can ensure their access to the Turkish Market. Exporters who wish to enter the Turkish market but missed the pre-registration should pre-register as soon as possible to be able to benefit from the advantages of joint registration.
KKDIK (aka Türkiye REACH) came into force on the 23rd of June 2017 and has replaced three existing regulations on chemicals and mixtures to regulate the chemical inventory, enforce secure usage, and risk management on hazardous substances, and promote alternatives to hazardous substances or procedures.
The regulation provides the means of registration, evaluation, authorization, and restrictions of chemicals and safeguarding human health and the environment. It also encourages using alternative methods for evaluating the hazard of substances such as decreasing the number of animal tests and encouraging innovation so that the competitiveness of the Turkish chemical industry will be enhanced.
KKDIK Timeline consists of 3 main phases:
- Pre-registration (Deadline has passed)
- Registration (Different deadlines for different tonnage bands)
- Post-registration
Türkiye Reach (KKDIK) Registration Phase Began!
It is strongly recommended for exporters to Türkiye to submit late pre-registrations (LPR) as soon as possible to secure business if not done already before 31st December 2020. Pre-registration carries crucial importance to be able to actively participate in SIEF activities for joint registrations.
KKDIK allows only pre-registered substances to be placed in the Turkish market from the 1st of January 2021 and onwards. Pre-registration is a simple activity without major compliance requirements that also allows GPC to represent your best interest within the SIEF.
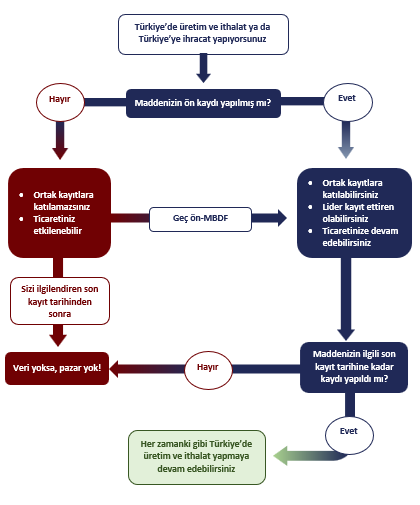
KKDIK is a comprehensive regulation. However, some substances are exempted from registration either because they are covered by specific regulations such as radioactive waste or regarded as less risky such as polymers. The registration process is not the same for every substance for instance under some conditions, registration of on-site or transported isolated intermediates is relatively easy.
Registration requirements change depending on the exported tonnage of the substance. When the tonnage increases, additional information is needed. For instance, when the tonnage of a substance is 10-100 tons per annum, a chemical safety report should be provided. Therefore, the best and easiest action would be to e-mail us your substance details and we will provide you with your customized complete obligations under Türkiye REACH.
Current Deadlines
The Ministry of Environment, Urbanization, and Climate Change of Türkiye announced new KKDIK deadlines for the registration process.
- Pre-registration under the KKDIK needs to be completed according to the timeline soon to be published by the Ministry in a circular or similar official communication.
- The tonnage bands for registration are like EU REACH (1-10; 10-100; 100-1000 and 1000+ tons/year for full registration and <1000 or >1000 tons/year for intermediate registration).
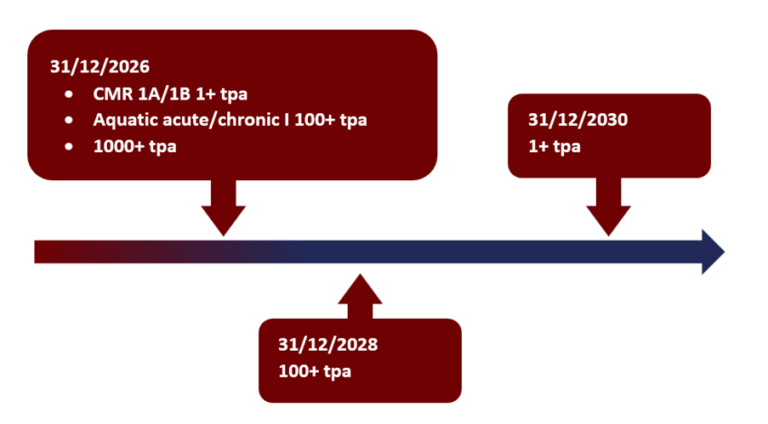
- The deadline for the final registration within KKDIK depends on the tonnage band and substance classification:
- 31st December 2026: 1000+ tons/year, 100+ tons/year if the substance is aquatic chronic/acute 1, 1+ tons/year if the substance is CMR 1A/B
- 31st December 2028: 100+ tons/year
- 31st December 2030: 1+ tons/year
Joint Registration
Joint registration aims to decrease the cost of registration by splitting the fee etc. and reduce the number of animal tests. Lead registrants, who are appointed by the SIEF members for each substance, prepare and submit the joint registration dossier on behalf of the SIEF. GPC can undertake the lead registrant role upon your request. Having managed approximately 1200 registration dossiers including 400 lead registrations in EU REACH, GPC is the holder of data for many substances and thus, we offer you the advantage of having a much faster & cost-effective registration process for the SIEFs that we are the lead of.




