UK Chemical Regulation
UK REACH
What is UK-REACH?
The EU REACH Regulation was brought into UK law, as amended by the REACH (EU Exit) Regulations 2019, on 1st January 2021 and is now known as UK REACH. UK REACH will now regulate the market access to Great Britain (GB) i.e. England, Scotland and Wales.
UK REACH has been described as mirroring its EU counterpart. With the aim of assisting the many exporters likely to be affected by the repeal of EU REACH in the Great Britain, UK REACH provides comprehensive transitional arrangements. Such arrangements concern both EU/EEA and non-EU/EEA based exporters dealing with substances which are already registered under EU REACH and now wishing to maintain access to the GB. Transitional provisions are, however, not applicable to those who will place the substances for the first time in the GB market.
In order to secure your access to the GB market, you should consider getting in touch with a GB-based OR without delay.
GPC Group has already set up a UK-based OR, to support our existing and new clients, in addressing the challenges posed by Brexit on substance exports to the UK.
Brexit Current Status
EU REACH has ceased to apply in Great Britain on January 1st 2021, as a consequence of the United Kingdom’s effective withdrawal from the European Union. GB manufacturers, importers, distributors and downstream users of chemical substances must now comply with UK REACH, the UK regulation adopted in replacement of EU REACH.
Who is concerned?
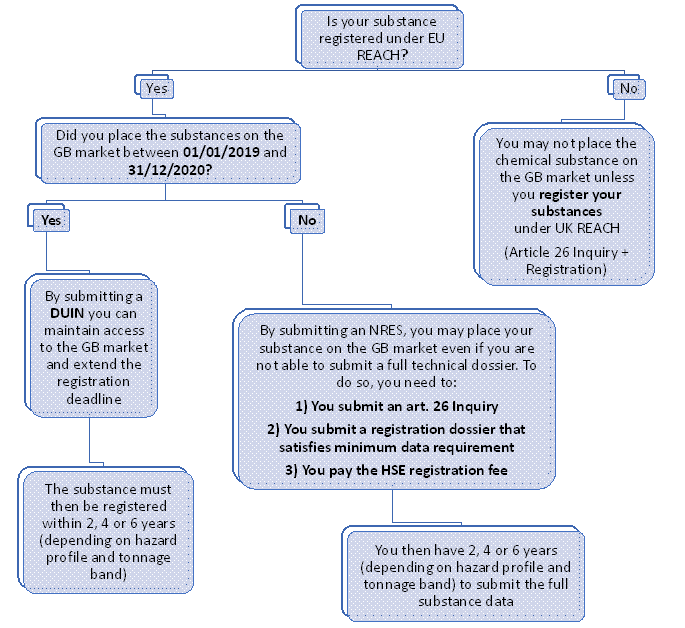
Companies seeking to export an EU-registered substance to the GB market, who also placed that substance on the GB market in 2019-2020:
Such exporters may submit a Downstream User Import Notification (DUIN) to HSE. By notifying their substance via DUIN, exporters will maintain access to the GB-market while deferring the registration of their substance by 2, 4 or 6 years (depending on the tonnage band and risk profile of the substance).
To submit a DUIN, non-GB based exporters need to appoint a GB-based Only Representative. The deadline for DUIN expired on 27 October 2021, however late-DUINs can still be submitted.
Companies seeking to export an EU-registered substance to the GB market for the first time
These exporters may benefit form a simplified registration process named New Registration of an Existing Substance (NRES). NRES requires exporters to:
- Submit an Inquiry
- Pay HSE’s registration fee
- Submit a simplified registration dossier (which does not need to include the LoA)
Once these steps have been completed the substance can be placed on the GB-market. The full registration dossier will have to be submitted within 2, 4 or 6 years form 27 October 2021 (depending on the tonnage band and risk profile of the substance)
Please note that NRES only applies to substances that were registered under EU REACH before 31st December 2020.
Exporters of a non-EU-registered substance wishing to access the GB market:
Exporters who do not qualify for DUIN or NRES must register their substances under UK-REACH before they may place that substance on the GB-market. Non-GB based exporters must appoint a GB-based OR to complete the full registration of the substance. To register, exporters need to
- Submit an Inquiry
- Pay HSE’s registration fee
- Submit a full registration dossier (including the LoA)
Which procedure applies to your business?
Actionable Summary for Chemical Businesses
- Identify the substances to be placed in the GB market in qty. ≥ 1 tpa apart from the EU exports earlier
- Check- if the substance is already registered in EU-REACH as EU Registered substances make you eligible for DUIN
- Check- if the substance will be placed in the GB for the first time – Submit inquiry and registration dossier as soon as possible
- Early submission means possibility to be a part of substance group so prioritise substances for inquiry submission immediately after DUIN
- Check- if the SDS is updated or not – the substance classification should be as per the new GB CLP
- Identify the competent OR to take responsibilities towards your UK-REACH compliances.
- Be transparent to your OR and give complete information to avoid any non-compliances




